Unit chemical reactions balancing equations – wksh #2 answer key – Embark on a journey to master the art of balancing chemical equations with our comprehensive guide, the Unit Chemical Reactions Balancing Equations – Worksheet #2 Answer Key. This essential resource provides a thorough understanding of the fundamental principles and practical applications of balancing equations, empowering you to navigate the complexities of chemical reactions with confidence.
Delve into the intricacies of balancing chemical equations, exploring the significance of coefficients and the step-by-step methods used to achieve balanced equations. Discover the nuances of unit chemical reactions, categorizing them into synthesis, decomposition, single-replacement, and double-replacement reactions. Engage with interactive practice exercises, honing your skills in balancing equations of varying types and difficulty levels.
Balancing Chemical Equations
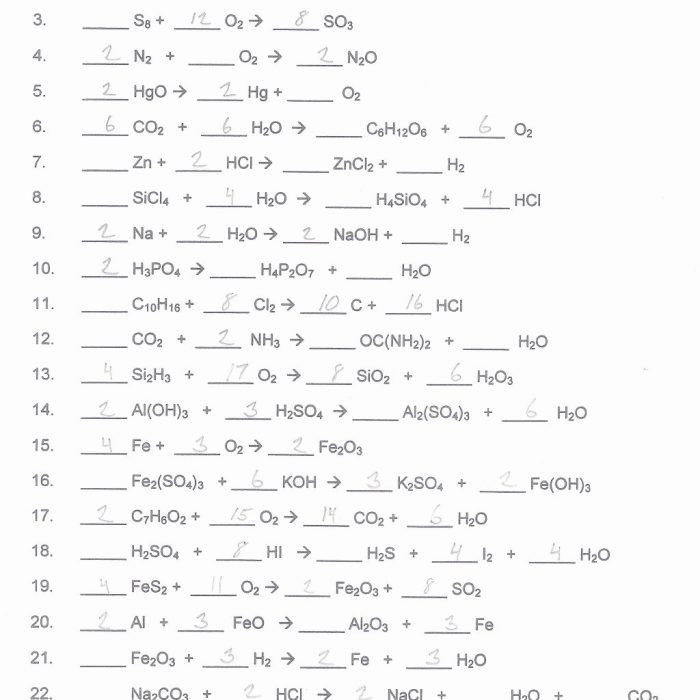
Balancing chemical equations is a fundamental skill in chemistry. It ensures that the number of atoms of each element on the reactants’ side of an equation equals the number of atoms of that element on the products’ side. This is important because chemical reactions must obey the law of conservation of mass, which states that matter cannot be created or destroyed.
To balance an equation, coefficients are added to the reactants and products. Coefficients represent the number of molecules or moles of each substance involved in the reaction. Balancing equations can be done using various methods, such as the oxidation-reduction method or the half-reaction method.
Unit Chemical Reactions
Unit chemical reactions are the simplest type of chemical reactions. They involve only one reactant and one product. Unit chemical reactions can be classified into four types: synthesis, decomposition, single-replacement, and double-replacement.
- Synthesis reactions combine two or more substances to form a single product.
- Decomposition reactions break down a single substance into two or more products.
- Single-replacement reactions involve one element replacing another element in a compound.
- Double-replacement reactions involve two compounds exchanging ions to form two new compounds.
Worksheet #2 Answer Key, Unit chemical reactions balancing equations – wksh #2 answer key
| Reaction | Balanced Equation | Type of Reaction | Notes |
|---|---|---|---|
| 2Na + 2H2O → 2NaOH + H2 | 2Na + 2H2O → 2NaOH + H2 | Single-replacement | Sodium replaces hydrogen in water. |
| Fe2O3 + 3CO → 2Fe + 3CO2 | Fe2O3 + 3CO → 2Fe + 3CO2 | Redox | Carbon monoxide reduces iron oxide to iron. |
| 2NH3 + 3CuO → 3Cu + N2 + 3H2O | 2NH3 + 3CuO → 3Cu + N2 + 3H2O | Redox | Ammonia reduces copper oxide to copper. |
| CaCO3 → CaO + CO2 | CaCO3 → CaO + CO2 | Decomposition | Calcium carbonate decomposes into calcium oxide and carbon dioxide. |
Practice Exercises
- Balance the following equation: Fe + HCl → FeCl2+ H 2
- Balance the following equation: MgO + HCl → MgCl 2+ H 2O
- Balance the following equation: C 6H 12O 6+ O 2→ CO 2+ H 2O
Real-World Applications
Balancing chemical equations has numerous real-world applications. It is essential in industrial chemistry for designing and optimizing chemical processes. In environmental science, it helps determine the stoichiometry of reactions involved in pollution control and remediation. In medicine, it is used to calculate drug dosages and understand the metabolism of drugs in the body.
FAQ Compilation: Unit Chemical Reactions Balancing Equations – Wksh #2 Answer Key
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation matches the number of atoms of that element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate stoichiometric calculations and a deep understanding of chemical reactions.
How do I approach balancing chemical equations?
Begin by identifying the unbalanced equation and ensuring that all reactants and products are represented. Then, start balancing the elements one at a time, adjusting the stoichiometric coefficients in front of each chemical formula until the number of atoms of each element is equal on both sides of the equation.
What types of chemical reactions are covered in this guide?
This guide encompasses the four main types of unit chemical reactions: synthesis, decomposition, single-replacement, and double-replacement reactions. Each type is thoroughly explained, with examples and practice exercises to reinforce your understanding.