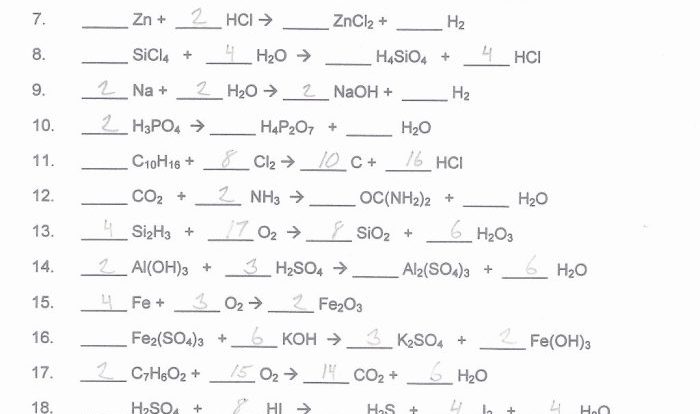
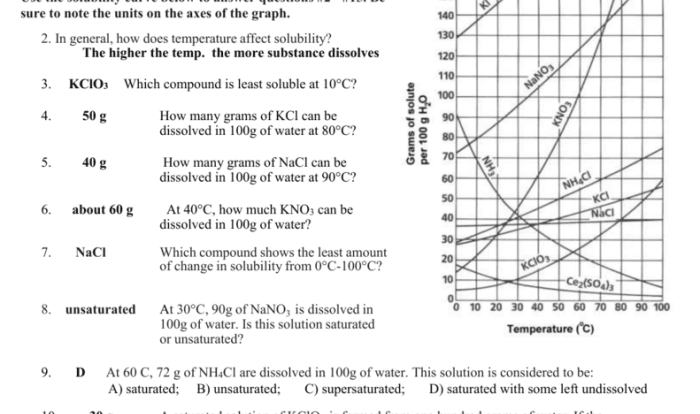
Embarking on an academic expedition, we delve into the realm of student exploration: polarity and intermolecular forces. This journey will illuminate the fundamental concepts that govern molecular interactions, shaping the physical properties and behaviors of substances that surround us.
Polarity, stemming from electronegativity differences, dictates the distribution of charge within molecules, influencing their reactivity and solubility. Intermolecular forces, ranging from dipole-dipole interactions to hydrogen bonding, determine the strength of intermolecular attractions, impacting melting and boiling points, surface tension, and capillary action.
Student Exploration of Polarity
Polarity in chemistry refers to the separation of electric charge within a molecule or chemical species. It arises when electrons are not shared equally between atoms, resulting in a partial positive charge on one atom and a partial negative charge on another.
Electronegativity, a measure of an atom’s ability to attract electrons, plays a crucial role in determining polarity. Atoms with higher electronegativity tend to attract electrons more strongly, leading to a greater separation of charge and increased polarity.
Polar molecules possess a permanent dipole moment due to the uneven distribution of electrons. Examples include water (H2O), ammonia (NH3), and hydrogen chloride (HCl). Nonpolar molecules, on the other hand, have electrons distributed symmetrically, resulting in no net dipole moment.
Examples include methane (CH4), carbon dioxide (CO2), and benzene (C6H6).
Intermolecular Forces and Their Effects
Intermolecular forces are attractive or repulsive forces that act between molecules. They include van der Waals forces, dipole-dipole interactions, and hydrogen bonding. Van der Waals forces are weak attractive forces that exist between all molecules, regardless of their polarity. Dipole-dipole interactions are stronger attractive forces that occur between polar molecules with permanent dipole moments.
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
Intermolecular forces have a significant impact on the physical properties of substances. Substances with strong intermolecular forces tend to have higher melting points and boiling points because more energy is required to overcome these forces and separate the molecules. For example, water has a relatively high boiling point (100°C) due to the strong hydrogen bonding between water molecules.
Impact of Polarity and Intermolecular Forces on Everyday Phenomena
Polarity and intermolecular forces play a crucial role in various everyday phenomena.
- Solubility: Polar substances tend to dissolve in polar solvents, while nonpolar substances tend to dissolve in nonpolar solvents. This is because the intermolecular forces between the solute and solvent must be similar in strength for dissolution to occur.
- Surface tension and capillary action: Surface tension is a measure of the force required to break the surface of a liquid. Polar liquids have higher surface tension than nonpolar liquids because of the stronger intermolecular forces between the polar molecules. Capillary action is the ability of a liquid to flow upward through a narrow tube.
It is caused by the attraction between the liquid molecules and the walls of the tube.
Design an Experiment to Investigate Polarity and Intermolecular Forces: Student Exploration: Polarity And Intermolecular Forces

An experiment to determine the polarity of a given substance can involve measuring its solubility in different solvents. A polar substance will dissolve more readily in a polar solvent than in a nonpolar solvent. Alternatively, the polarity of a substance can be inferred from its physical properties, such as its melting point and boiling point.
The following table Artikels an experiment to investigate the polarity of a substance:
| Substance | Solubility in water (polar solvent) | Solubility in hexane (nonpolar solvent) | Melting point (°C) | Boiling point (°C) |
|---|---|---|---|---|
| Water (H2O) | Soluble | Insoluble | 0 | 100 |
| Hexane (C6H14) | Insoluble | Soluble | -95 | 69 |
| Ethanol (C2H5OH) | Soluble | Soluble | -114 | 78 |
| Sodium chloride (NaCl) | Soluble | Insoluble | 801 | 1465 |
By comparing the solubility and physical properties of the substances, it is possible to determine their polarity. For example, water is a polar substance because it is soluble in water and has a relatively high melting point and boiling point.
Hexane is a nonpolar substance because it is insoluble in water and has a relatively low melting point and boiling point.
Question Bank
What is the significance of polarity in molecular interactions?
Polarity determines the charge distribution within molecules, influencing their reactivity and solubility. Polar molecules possess a partial positive and negative charge, enabling them to interact with other polar molecules or ions.
How do intermolecular forces affect the physical properties of substances?
Intermolecular forces dictate the strength of intermolecular attractions, which in turn influences physical properties such as melting point, boiling point, surface tension, and capillary action. Stronger intermolecular forces lead to higher melting and boiling points, as more energy is required to overcome these attractions.